Template:Infobox helium
Jump to navigation
Jump to search
[[Category:Template:Pagetype with short description]]
 | ||||||||||||||||
| Helium | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /ˈhiːliəm/ | |||||||||||||||
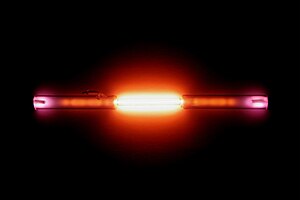
| Appearance | colorless gas, exhibiting a gray, cloudy glow (or reddish-orange if an especially high voltage is used) when placed in an electric field | |||||||||||||||
| Standard atomic weight Ar, std(He) | Template:Val[1] | |||||||||||||||
| Helium in the periodic table | ||||||||||||||||
| ||||||||||||||||
| Atomic number (Z) | 2 | |||||||||||||||
| Group | group 18 (noble gases) | |||||||||||||||
| Period | period 1 | |||||||||||||||
| Block | s-block | |||||||||||||||
| Electron configuration | 1s2 | |||||||||||||||
| Electrons per shell | 2 | |||||||||||||||
| Physical properties | ||||||||||||||||
| Phase at STP | gas | |||||||||||||||
| Melting point | 0.95 K (−272.20 °C, −457.96 °F) (at 2.5 MPa) | |||||||||||||||
| Boiling point | 4.222 K (−268.928 °C, −452.070 °F) | |||||||||||||||
| Density (at STP) | 0.1786 g/L | |||||||||||||||
| when liquid (at m.p.) | 0.145 g/cm3 | |||||||||||||||
| when liquid (at b.p.) | 0.125 g/cm3 | |||||||||||||||
| Triple point | 2.177 K, 5.043 kPa | |||||||||||||||
| Critical point | 5.1953 K, 0.22746 MPa | |||||||||||||||
| Heat of fusion | 0.0138 kJ/mol | |||||||||||||||
| Heat of vaporization | 0.0829 kJ/mol | |||||||||||||||
| Molar heat capacity | 20.78 J/(mol·K)[2] | |||||||||||||||
Vapor pressure (defined by ITS-90)
| ||||||||||||||||
| Atomic properties | ||||||||||||||||
| Oxidation states | 0 | |||||||||||||||
| Electronegativity | Pauling scale: no data | |||||||||||||||
| Ionization energies |
| |||||||||||||||
| Covalent radius | 28 pm | |||||||||||||||
| Van der Waals radius | 140 pm | |||||||||||||||
| Spectral lines of helium | ||||||||||||||||
| Other properties | ||||||||||||||||
| Natural occurrence | primordial | |||||||||||||||
| Crystal structure | hexagonal close-packed (hcp) | |||||||||||||||
| Speed of sound | 972 m/s | |||||||||||||||
| Thermal conductivity | 0.1513 W/(m·K) | |||||||||||||||
| Magnetic ordering | diamagnetic[3] | |||||||||||||||
| Magnetic susceptibility | −1.88·10−6 cm3/mol (298 K)[4] | |||||||||||||||
| CAS Number | 7440-59-7 | |||||||||||||||
| History | ||||||||||||||||
| Naming | after Helios, Greek god of the Sun | |||||||||||||||
| Discovery | Pierre Janssen, Norman Lockyer (1868) | |||||||||||||||
| First isolation | William Ramsay, Per Teodor Cleve, Abraham Langlet (1895) | |||||||||||||||
| Main isotopes of helium | ||||||||||||||||
| ||||||||||||||||
[[Category:Infobox templates|Template:Remove first word]]
| data m.p. cat | |||||
|---|---|---|---|---|---|
| in | calc from C | diff | report | ref | |
| C | −272.20 | — | — | ||
| K | 0.95 | 0.950 | 0 | ||
| F | −457.96 | −457.960 | 0 | Template:Subpage other | |
| max precision | 2 | ||||
| WD | Template:Wikidata Script error: No such module "EditAtWikidata". | Template:Wikidata | |||
| input | C: −272.20, K: 0.95, F: −457.96 | ||||
| comment | (at 2.5 MPa) | ||||
| data b.p. cat | |||||
|---|---|---|---|---|---|
| in | calc from C | diff | report | ref | |
| C | −268.928 | — | — | ||
| K | 4.222 | 4.222 | 0 | ||
| F | −452.070 | −452.070 | 0 | Template:Subpage other | |
| max precision | 3 | ||||
| WD | Template:Wikidata Script error: No such module "EditAtWikidata". | Template:Wikidata | |||
| input | C: −268.928, K: 4.222, F: −452.070 | ||||
| comment | |||||
| H ← |
→ Li | |
| ||
| Data sets read by {{Infobox element}} | |
|---|---|
| Name and identifiers | |
| Top image (caption, alt) | |
| Pronunciation | |
| Category (enwiki) | |
| Standard atomic weight | |
| most stable isotope | |
| Natural occurrence | |
| Phase at STP | |
| Oxidation states | |
| Spectral lines image | |
| Electron configuration (cmt, ref) | |
| Term symbol * (cmt, ref) | |
| Wikidata * | |
| * Not used in {{Infobox element}} (2019-02-03) See also {{Infobox element/symbol-to--navbox}} | |
References
- ↑ Meija, Juris; et al. (2016). "Atomic weights of the elements 2013 (IUPAC Technical Report)". Pure and Applied Chemistry. 88 (3): 265–91. doi:10.1515/pac-2015-0305.
- ↑ Shuen-Chen Hwang, Robert D. Lein, Daniel A. Morgan (2005). "Noble Gases". Kirk Othmer Encyclopedia of Chemical Technology. Wiley. pp. 343–383. doi:10.1002/0471238961.0701190508230114.a01.
- ↑ Magnetic susceptibility of the elements and inorganic compounds, in Handbook of Chemistry and Physics 81st edition, CRC press.
- ↑ Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 0-8493-0464-4.
One of these is a named reference. It may be cited in the containing article as
- <ref name = "CIAAW2013" /> for the source Atomic weights of the elements 2013 (from subtemplates used by {{Infobox element}})
Lua error: Internal error: The interpreter has terminated with signal "24". +