Template:Infobox francium/sandbox
Jump to navigation
Jump to search
[[Category:Template:Pagetype with short description]]
| Francium | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /ˈfrænsiəm/ | ||||||||||||||||||||||
| Mass number | [223] | ||||||||||||||||||||||
| Francium in the periodic table | |||||||||||||||||||||||
| |||||||||||||||||||||||
| Atomic number (Z) | 87 | ||||||||||||||||||||||
| Group | group 1: H and alkali metals | ||||||||||||||||||||||
| Period | period 7 | ||||||||||||||||||||||
| Block | s-block | ||||||||||||||||||||||
| Electron configuration | [Rn] 7s1 | ||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 18, 8, 1 | ||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||
| Phase at STP | solid presumably | ||||||||||||||||||||||
| Melting point | ? 300 K (27 °C, 80 °F) | ||||||||||||||||||||||
| Boiling point | ? 950 K (677 °C, 1250 °F) | ||||||||||||||||||||||
| Density (near r.t.) | 1.87 g/cm3 (extrapolated) | ||||||||||||||||||||||
| Heat of fusion | ca. 2 kJ/mol | ||||||||||||||||||||||
| Heat of vaporization | ca. 65 kJ/mol | ||||||||||||||||||||||
Vapor pressure (extrapolated)
| |||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||
| Oxidation states | +1 (a strongly basic oxide) | ||||||||||||||||||||||
| Electronegativity | Pauling scale: 0.7 | ||||||||||||||||||||||
| Ionization energies |
| ||||||||||||||||||||||
| Covalent radius | 260 pm (extrapolated) | ||||||||||||||||||||||
| Van der Waals radius | 348 pm (extrapolated) | ||||||||||||||||||||||
| Other properties | |||||||||||||||||||||||
| Natural occurrence | from decay | ||||||||||||||||||||||
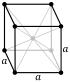
| Crystal structure | body-centered cubic (bcc) (extrapolated) | ||||||||||||||||||||||
| Thermal conductivity | 15 W/(m·K) (extrapolated) | ||||||||||||||||||||||
| Electrical resistivity | 3 µΩ·m (calculated) | ||||||||||||||||||||||
| Magnetic ordering | Paramagnetic | ||||||||||||||||||||||
| CAS Number | 7440-73-5 | ||||||||||||||||||||||
| History | |||||||||||||||||||||||
| Naming | after France, homeland of the discoverer | ||||||||||||||||||||||
| Discovery and first isolation | Marguerite Perey (1939) | ||||||||||||||||||||||
| Main isotopes of francium | |||||||||||||||||||||||
| |||||||||||||||||||||||
References
Lua error: Internal error: The interpreter has terminated with signal "24".