Revision as of 14:34, 5 October 2019 by imported>DePiep
[[Category:Template:Pagetype with short description]]
Cobalt, 27 Cobalt Pronunciation [1] Appearance hard lustrous bluish gray metal Standard atomic weight A r, std (Co) Template:Val [2] Cobalt in the periodic table
Atomic number (Z ) 27 Group group 9 Period period 4 Block d-block Electron configuration [Ar ] 3d7 4s2 Electrons per shell 2, 8, 15, 2 Physical properties Phase at STP solid Melting point 1768 K (1495 °C, 2723 °F) Boiling point 3200 K (2927 °C, 5301 °F) Density (near r.t. ) 8.90 g/cm3 when liquid (at m.p. ) 8.86 g/cm3 Heat of fusion 16.06 kJ/mol Heat of vaporization 377 kJ/mol Molar heat capacity 24.81 J/(mol·K) Vapor pressure
P (Pa)
1
10
100
1 k
10 k
100 k
at T (K)
1790
1960
2165
2423
2755
3198
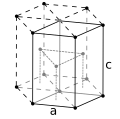
Atomic properties Oxidation states −3, −1, 0, +1, +2 +3 [3] amphoteric oxide) Electronegativity Pauling scale: 1.88 Ionization energies 1st: 760.4 kJ/mol 2nd: 1648 kJ/mol 3rd: 3232 kJ/mol (more ) Atomic radius empirical: 125 pm Covalent radius Low spin: 126±3 pm Spectral lines of cobaltOther properties Natural occurrence primordial Crystal structure hexagonal close-packed (hcp) Speed of sound thin rod 4720 m/s (at 20 °C) Thermal expansion 13.0 µm/(m·K) (at 25 °C) Thermal conductivity 100 W/(m·K) Electrical resistivity 62.4 nΩ·m (at 20 °C) Magnetic ordering ferromagnetic Young's modulus 209 GPa Shear modulus 75 GPa Bulk modulus 180 GPa Poisson ratio 0.31 Mohs hardness 5.0 Vickers hardness 1043 MPa Brinell hardness 470–3000 MPa CAS Number 7440-48-4 History Discovery and first isolationGeorg Brandt (1735)Main isotopes of cobalt
Category: Cobalt references
[[Category:Infobox templates|Template:Remove first word ]]
References These references will appear in the article, but this list appears only on this page.
Lua error: Internal error: The interpreter has terminated with signal "24".